MKT 0251 Pilot Study
ABSTRACT
objective:
Prospective, multicenter, phase ll, non-randomized clinical study to evaluate the effectiveness and safety of the Axiom Worldwide DRX9000® tm for active treatment of chronic LBP utillizing a standardized clinical research multimodal protocal.
METHODS:
20 patients with chionic LBP, based on a diagnosis of musculoskeletal or mechanical LBP, herniated discs, bulging or protruding discs, degenerative disc, pain from failed back surgery more then 6 months previously, posterior facet syndrome or sciatica underwent a series of 20 DRX treatments (28 mins each) for weeks with 5 sessions the first week tapering to 1 sessions, lumbar stretching exerciser and adjunct analgesic as required. Assessments of pain, analgesic use functionality, satisfaction, activities of daily living and safety were collected through examinations, questionnaires and patient diaries.
RESULTS:
18 evaluable subjects (33.3% female,83.3% white, mean age46.6,77.8% employed) had mean pain score 6.4 on a 0 to 10 scale (0=no pain 10=worst pain) Prior to first DRX treatment that decreased to 0. after last DRX treatment 88.9% of patients (16 outof 18) reported an improvement in back pain, and better function as measured by activities of daily living. on a 0 to 10 scale (0=Not satisfied 10=very satisfed) patients rated the DRX9000® an 8.1. no patient required any invasive therapies (e.g, eqidural injection, surgery).
CONCLUSION:
overall, patients pain improved after DRX treatment requiring fewer analgesics, with better function. there were no safety issues identified with the multimodal treatment routine.Non-treatment or control groups were not included making efficacy outcome versus placebr or spontaneous recovery difficult to determine. randomized double-blinded or comparative long-term outcome trials are needed to further prove the efficacy of the DRX9000® non surgical spinal decopression system for the routine treatment of chronic LBP.
- Paucity of literature on benefits of none-surgical spinal decompression over other non-surgicl treatments
- Previous studies are poorly desigend
- Results are descriptive in nature
- Efficacy versues placebo or spontaneous recovery difficult to determine
- Over 1200 DRX9000® in use today
MATERIALS AND METHODS
METHODS
- Prospectiive, multi-center, phase ll, non-randomized clinical trial
- 3 free-standing clinics (2 MDs and 1DC)
- Diagnosis:low back pain > 12 weeks
- Outcome measures assessed:
-Daily pain diary
-verbal rating scale(VRS)
-Oswestry pain questionnaire
-Adverse Events -Satisfaction survey
TREATMENT PROTOCOL
- DRX9000® sessions
-28-minute sessions for 6 weeks
-Tolal of 20 treatments
- 5 sessions weeks 1&2
- 3 sessions weeks 3&4
- 2 sessions weeks 5&6
- Additional Therapy
-ice therapy post DRX
-Back exercises after week 2
RESULTS
| DEMOGRAPHICS |
|---|
| Total Number of Subjects=18 |
| Male | 66.7% | Mean Age | 46.6yrs |
| LBP symptom Duration(mean) | 526 weeks | Mean Height | 175 cm |
| Employed | 77.8% | Mean Weight | 102 Kg |
| Retired | 16.6% | White | 83.3% |
| Other | 5.6% | Hispanic | 16.7% |
| FAILED THERAPY PRIOR TO DRX9000® |
|---|
| Procedure | # | Procedure | # |
| Chiropractic | 16 | TENS | 5 |
| Muscle stimulation | 10 | Acupuncture | 3 |
| Ice Therapy | 9 | Lumbar Sopport | 3 |
| Massage Therapy | 9 | Epidural injections | 3 |
| Exercise | 6 | Facet injections | 1 |
| Heat | 5 | Ultrasound | 1 |
| Physical Therapy | 5 | Other Decom-pressive Therapy | 1 |
| FAILED THERAPY PRIOR TO DRX9000® | |||
| SUMMARY OF LOW BACK PAIN |
|---|
| DIAGNOSIS | LOCATION | ||
|---|---|---|---|
| Bulging/Protruding Disc | 15 | L1-L2 | 1 |
| Degenerative Disc | 8 | L2-L3 | 3 |
| Herniated Disc | 6 | L3-L4 | 4 |
| Posterior Facet Syndrome | 2 | L4-L5 | 14 |
| Failed Back Surgery | 1 | L5-S1 | 12 |
| ADVERSE EVENTS |
|---|
| Adverse | Related to device | Adverse Event | Retated to device |
| Neck Pain | Possibly | Shoulder Pain | No |
| Head Cold (2) | No | LBP/flu-like Symptoms | No |
| Sinus headache(2) | No | Vertigo | No |
| Sinus infection | No | Adrenal insufficiency | No |
RESULTS
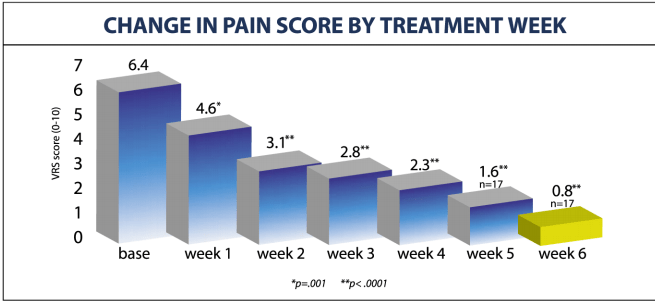
CHANGE PAIN SCORE BY TREATMENT WEEK
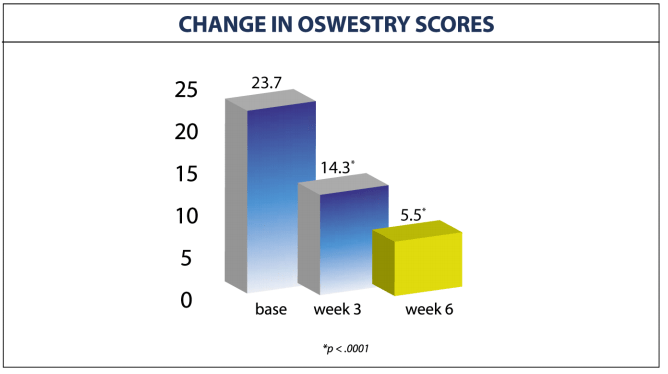
CHANGE IN OSWESTRY SCORES
| SATISFACTION SURVEY | |
|---|---|
| Satisfaction by week | Would you recommend DRX9000® to anyone else |
| Week 3 Week 6 7.6 8.1 | |
CONCLUSION
- A 6-week coures of 20 DRX9000® treatments significantly reduced the severity of chronic LBP in 89% (16 of 18) of treated patients form 6.4 to 3.1 after 2 week and to only 0.8 (scale 0-10) after completion of treatment
- Oswestry disability scores improved from 23.7 to only 5.5 at end of the therapy
- Adjunctive pain medication consumption was decreased by DRX9000® treatment
- No sideficant adverse events or safety issues resulted from DRX9000® treatment
- The DRX9000® shows great promise in treating chronic LBP arising from multiipal causes
- Comparative outcome trials utilizing a set of satndardized and validated multiple outcome variables as was utilized in this study are being planned to docment the value of DRX9000® non-surgical spinal decompression system in routine treatment of chronic LBP
